 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!

GMP Human FGF basic Protein (Cat. No. GMP-FGCH17) stimulates proliferation of NIH/3T3 cells. The specific activity of GMP Human FGF basic Protein is >2.50 x 10^6 IU/mg, which is calibrated against Basic Fibroblast Growth Factor WHO International Standard (NIBSC code: 90/712) (QC tested).
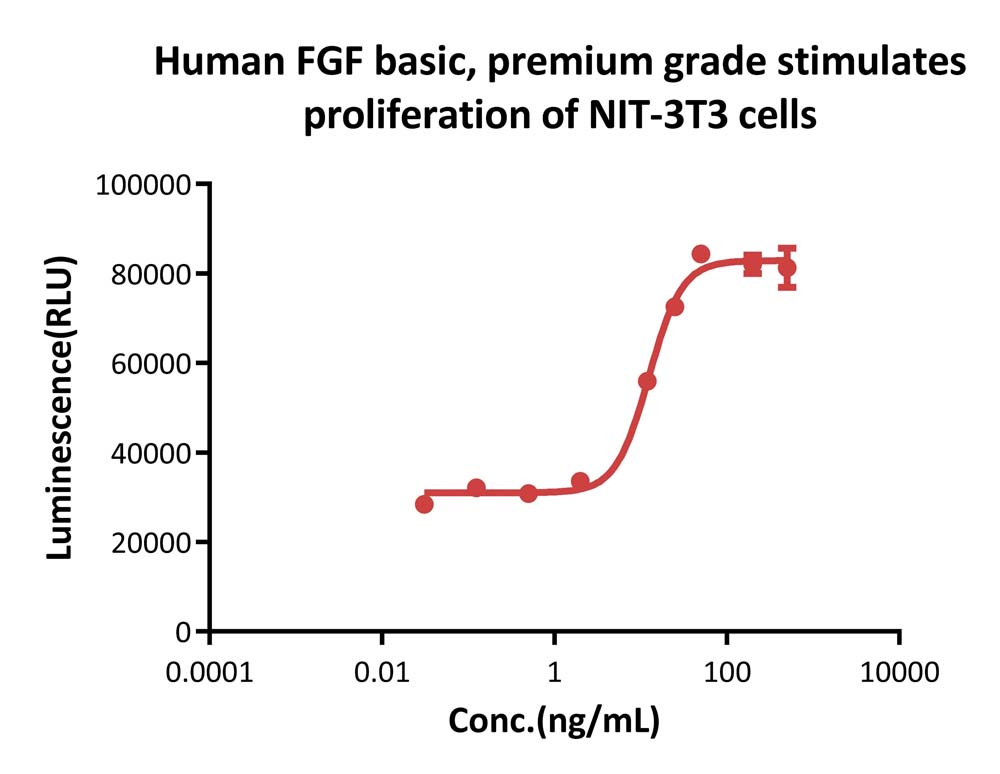
Human FGF basic, premium grade (Cat. No. BFF-H4117) stimulates proliferation of NIH/3T3 cells. The specific activity of Human FGF basic, premium grade is > 2.50×10^6 IU/mg, which is calibrated against human FGF basic WHO International Standard (NIBSC code: 90/712) (QC tested).
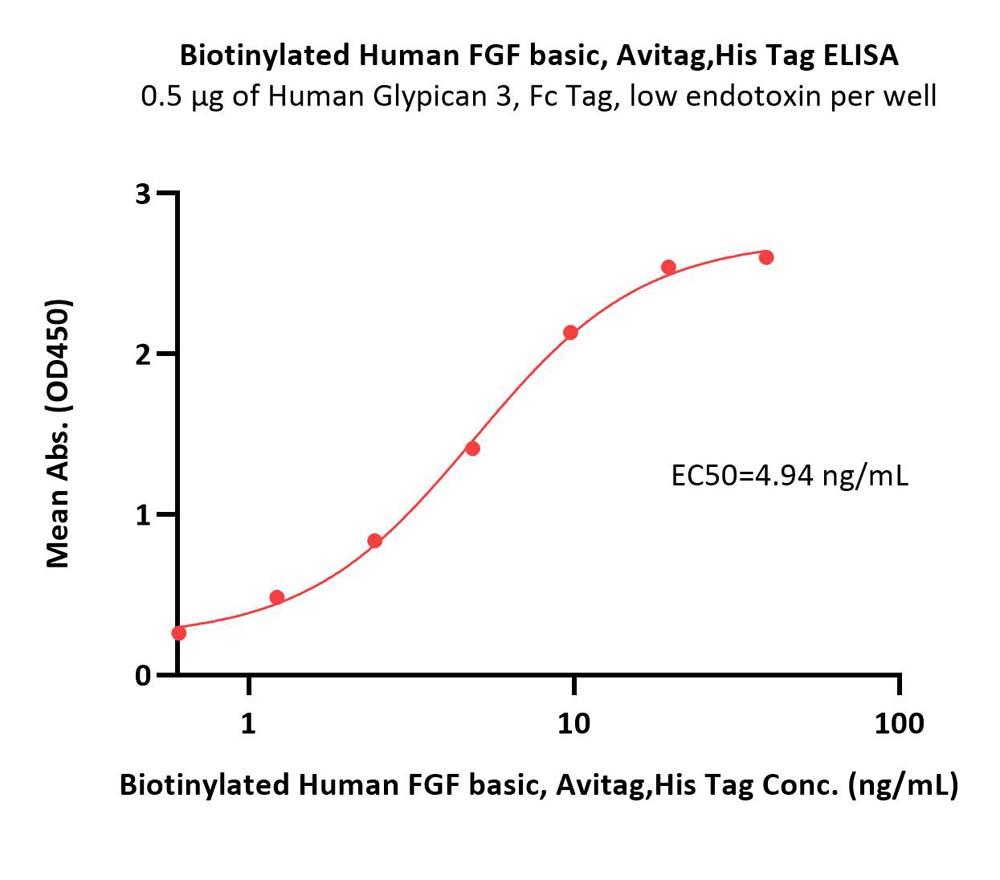
Immobilized Human Glypican 3, Fc Tag, low endotoxin (Cat. No. GP3-H5258) at 5 μg/mL (100 μL/well) can bind Biotinylated Human FGF basic, Avitag,His Tag (Cat. No. FGC-H81E3) with a linear range of 0.6-10 ng/mL (QC tested).
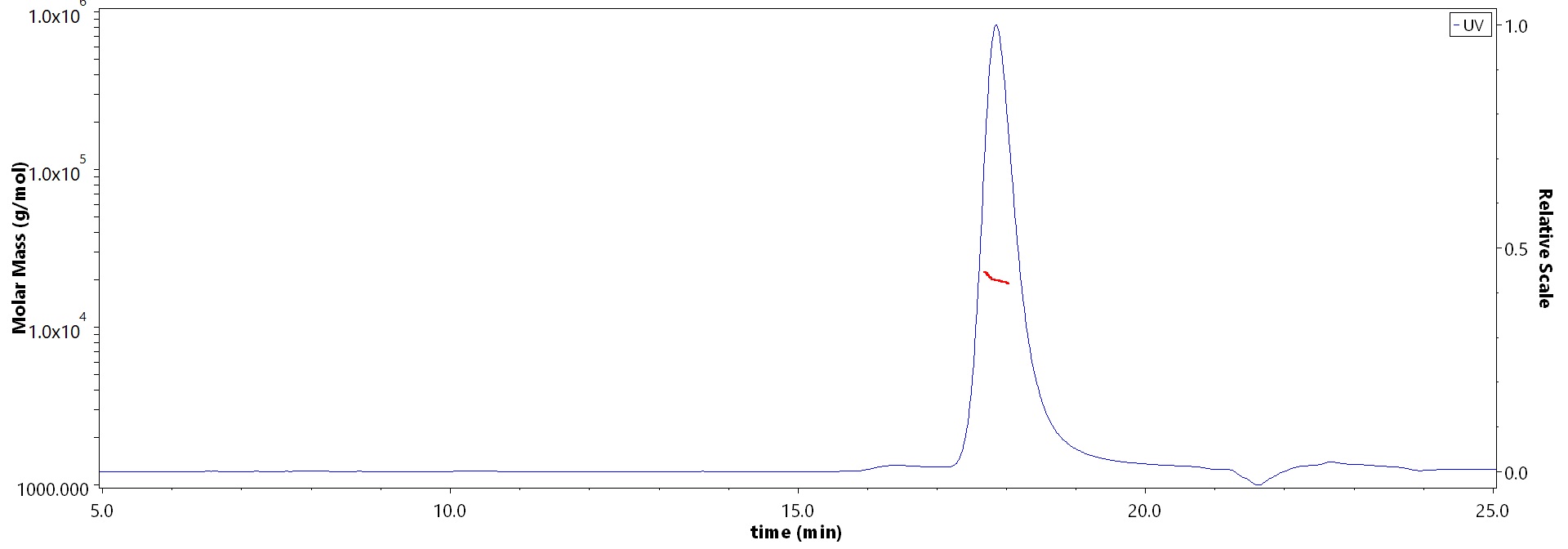
The purity of Human FGF basic (154aa) Protein, premium grade (Cat. No. BFF-H5115) is more than 90% and the molecular weight of this protein is around 15-25 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Thalidomide | NSC-66847; NSC-527179; K-17; VP-02; FPF-300; FPF300 | Approved | Celgene Corp | Talizer, Thalidomide Celgene, Thalidomide Pharmion, Synovir, Thalomid, Thaled | Mainland China | Leprosy, Lepromatous; Multiple Myeloma | Changzhou Pharmaceutical Factory | 1982-01-01 | HIV Wasting Syndrome; Angiodysplasia; Primary Myelofibrosis; Neuroectodermal Tumors, Primitive; Prostatitis; Colorectal Neoplasms; Osteosarcoma; Lymphoma, Mantle-Cell; Sarcoma, Ewing; Retinoblastoma; Erythema Nodosum; Drug Resistant Epilepsy; Xerostomia; Sarcoma; Pancreatitis, Chronic; Adenocarcinoma, Clear Cell; Lymphoma, Follicular; Arachnoiditis; Carcinoma, Adenosquamous; Gastrointestinal Hemorrhage; Cholangitis, Sclerosing; Prostatic Neoplasms; Pelvic Pain; Neoplasm Metastasis; Stomatitis; Burning Mouth Syndrome; Mycobacterium avium-intracellulare Infection; Amyotrophic Lateral Sclerosis; Melanoma; Myelodysplastic-Myeloproliferative Diseases; Carcinoma, Hepatocellular; Leukemia, Lymphocytic, Chronic, B-Cell; Vascular Malformations; Tuberculosis; Appendiceal Neoplasms; Lymphoma, Non-Hodgkin; Uterine Neoplasms; Anemia, Sideroblastic; Glioma; Leprosy, Lepromatous; Endometrial Neoplasms; Lung Neoplasms; Waldenstrom Macroglobulinemia; Kidney Neoplasms; Thalassemia; Carcinoid Tumor; Lupus Erythematosus, Discoid | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Muparfostat sodium | PI-88 | Phase 3 Clinical | Australian National University | Solid tumours; Liver Neoplasms; Neoplasms; Small Cell Lung Carcinoma; Prostatic Neoplasms; Lung Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| RBM-007 | ID3 (21); APT-F2; RBM-007 | Phase 2 Clinical | Ribomic | Achondroplasia; Macular Degeneration | Details |
| Fibroblast growth factor 2 gene therapy (ID Pharma) | SeV-10101; SEV-10101; DVC-10101; BF-30 | Phase 2 Clinical | Dnavec | Arterial Occlusive Diseases; Intermittent Claudication; Ischemia; Peripheral Arterial Disease; Peripheral arterial occlusive disorders | Details |
This web search service is supported by Google Inc.
